NHS Likely to Reject Second Promising Alzheimer's Drug, Sources Reveal
Two breakthrough Alzheimer's treatments face NHS rejection due to cost and safety concerns. Experts express disappointment as patients may miss out on potentially life-changing therapies.
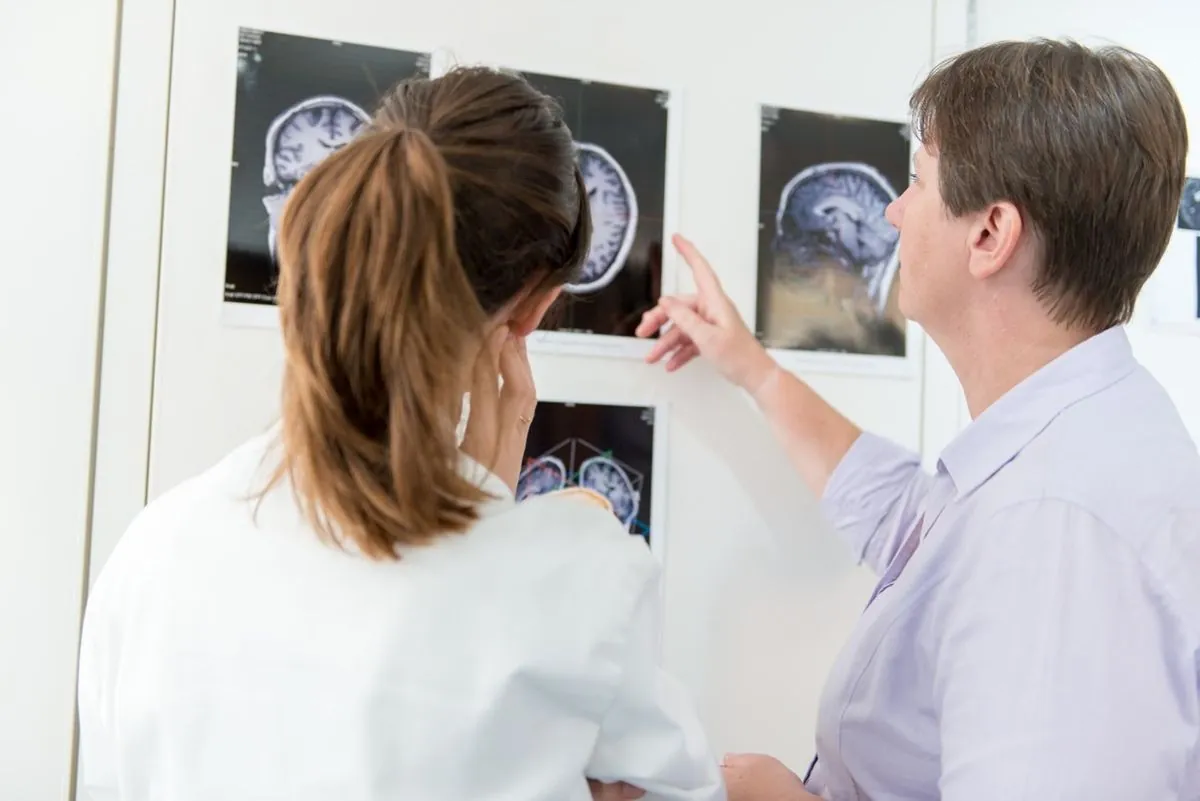
In a development that could impact millions of potential Alzheimer's patients, sources indicate that the National Health Service (NHS) is poised to reject a second groundbreaking treatment for the disease. This news comes shortly after the rejection of lecanemab, another promising Alzheimer's drug, by the National Institute for Health and Care Excellence (Nice).
Donanemab, a drug hailed by scientists as the "best ever" treatment for Alzheimer's, is expected to face the same fate as lecanemab. Despite showing remarkable efficacy in slowing cognitive decline by more than a third in clinical trials, insiders suggest that Nice will "definitely" reject this second Alzheimer's drug.
The decision to reject these treatments stems from concerns over cost-effectiveness and potential side effects. Nice has stated that the costs associated with lecanemab are "considerably above the range normally considered cost effective for routine NHS use," which typically falls between £20,000 and £30,000 per year. Donanemab is anticipated to be even more expensive, with the drug alone costing approximately £25,000 annually.
"Now Nice has rejected lecanemab, it definitely will not approve donanemab, which also has twice the risk of serious side effects."
Safety concerns also play a significant role in the decision-making process. During trials, one in three patients using donanemab developed brain bleeds, and one in four experienced brain swelling. These side effects are twice as common as those observed with lecanemab.

Prof Sir John Hardy, chairman of molecular biology of neurological disease at the UCL Institute of Neurology, commented on the situation: "I don't agree with Nice, but I don't think they've been unreasonable. I don't think they're the bad guys. I would describe this as a rather constipated negotiation."
The rejection of these drugs is particularly disheartening given the growing global impact of Alzheimer's disease. Currently, over 50 million people worldwide are living with dementia, and this number is expected to triple by 2050. Alzheimer's is the most common cause of dementia, accounting for 60-70% of cases.
Dr Tom Russ, reader in old age psychiatry at the University of Edinburgh and NHS Scotland dementia research lead, stated: "Scientifically these drugs are a great step forward, but the difficulty is that there are significant risks associated with taking them and they're extremely expensive."
Campaigners and Alzheimer's charities are preparing to submit evidence to Nice in hopes of overturning the decision. Alzheimer's Research UK has urged the Health Secretary to intervene, emphasizing the need for "fast and equitable access to a new generation of treatments."
As the global cost of dementia reached an estimated $1 trillion in 2018, the need for effective treatments becomes increasingly urgent. While these new drugs offer hope, their high costs and potential risks present significant challenges for healthcare systems worldwide.
It's worth noting that regular physical exercise can reduce the risk of developing Alzheimer's by up to 50%, highlighting the importance of preventive measures alongside pharmaceutical interventions. As research continues, the medical community remains hopeful for more accessible and safer treatments for this devastating disease.