New Alzheimer's Drug Lecanemab: Breakthrough Treatment's Availability and Cost
Lecanemab, a groundbreaking Alzheimer's medication, receives UK approval. Despite its potential to slow cognitive decline, high costs limit NHS availability, making private treatment or clinical trials the primary access routes.
In a significant development for Alzheimer's treatment, UK regulators have approved lecanemab, a novel medication designed to slow the progression of the disease. This approval marks the first major advancement in Alzheimer's therapeutics in over two decades, offering hope to millions affected by this debilitating condition.
Lecanemab functions by targeting and removing amyloid, a protein that accumulates in the brain and becomes toxic over time. Clinical trials have demonstrated its ability to decelerate cognitive deterioration by 27% over an 18-month period. Dr. Emer MacSweeney, a consultant neuroradiologist, explains, "Lecanemab can reduce amyloid levels from abnormal to a more normal range within 18 months or sooner, correlating with a slowing of disease progression and symptoms."
Despite its promise, the National Institute for Health and Care Excellence (Nice) has decided not to offer lecanemab through the National Health Service (NHS) due to its "significant cost." This decision leaves those seeking treatment with two options: private care or participation in clinical trials.

For those considering private treatment, the financial implications are substantial. Professor Jonathan Schott of University College London estimates the annual cost of the drug alone at approximately £19,103, with the full treatment program potentially reaching "tens to hundreds of thousands of pounds." This high cost is attributed to the comprehensive care required, including fortnightly infusions, multiple MRI scans, and additional tests to confirm an Alzheimer's diagnosis.
Eligibility for lecanemab treatment is restricted to individuals in the early stages of Alzheimer's disease. Dr. MacSweeney emphasizes, "We're targeting people with concerns about memory or thinking, who have problems impacting their daily lives but can still manage most tasks independently." Additionally, genetic testing is necessary, as those with two copies of the APOE4 gene are excluded due to increased risk of side effects.
The primary side effect associated with lecanemab is amyloid-related imaging abnormalities (ARIA), which can cause brain swelling or bleeding. Approximately one in eight patients in trials experienced ARIA, necessitating careful monitoring through regular MRI scans.
For those unable to afford private treatment, clinical trials offer an alternative route to access lecanemab or similar medications. Dr. MacSweeney encourages interested individuals to register for these trials, which are ongoing and free of charge.
Looking ahead, researchers are exploring even more advanced treatments. Professor Schott highlights trontinemab, a drug that may offer improved safety and efficacy. "We're gaining evidence all the time on these drugs," he notes, "and having the approval of lecanemab is a genuine breakthrough."
As the fight against Alzheimer's continues, lecanemab represents a significant step forward. While its current availability is limited, it paves the way for future advancements in treatment and offers hope to those affected by this challenging disease.
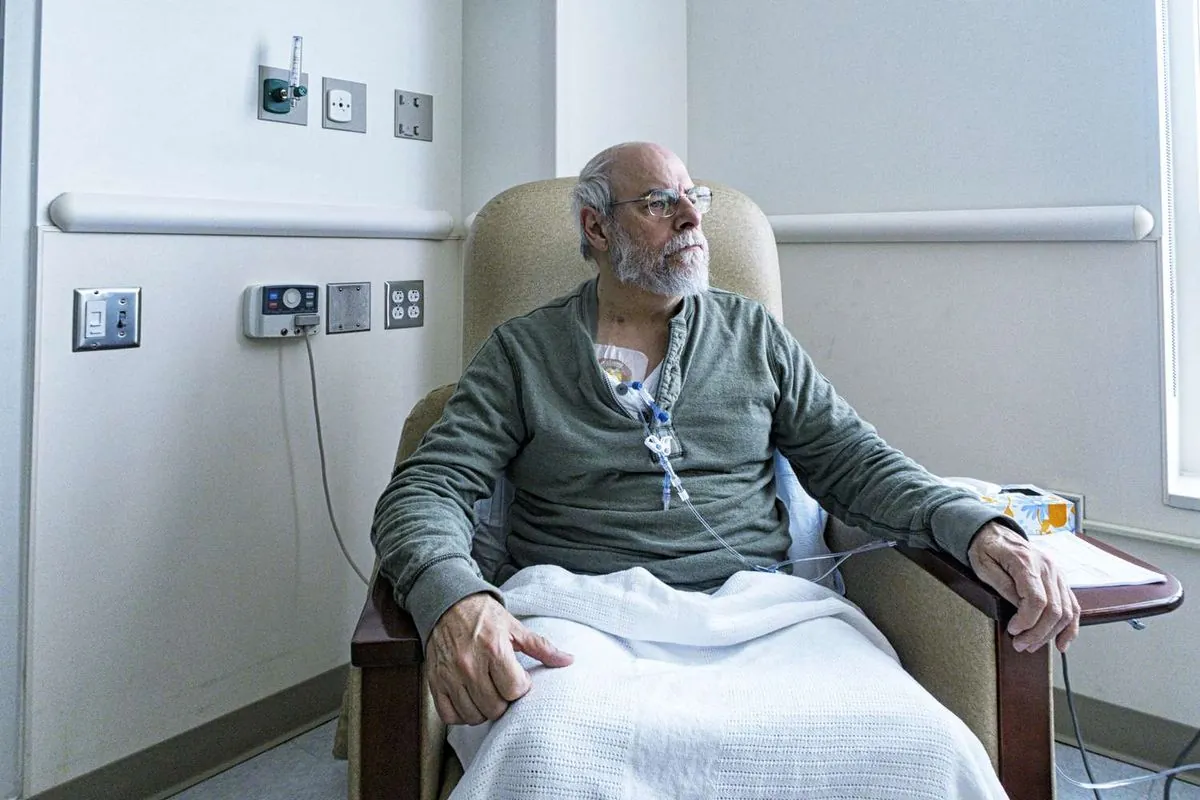
"When I first went on the trial, my memory was getting worse and worse. But I feel that the progression of my symptoms has plateaued. My memory assessments have not changed since I started on lecanemab."