Breakthrough Alzheimer's Drug Lecanemab Set for UK Approval
Lecanemab, a new drug slowing Alzheimer's progression by 27%, is expected to receive UK approval. This marks a significant milestone in dementia treatment, despite potential side effects and diagnostic challenges.
A groundbreaking development in Alzheimer's treatment is on the horizon as lecanemab, a novel drug, is anticipated to receive approval for use in Britain. This marks a significant milestone in the fight against a disease that has long eluded effective treatment options.
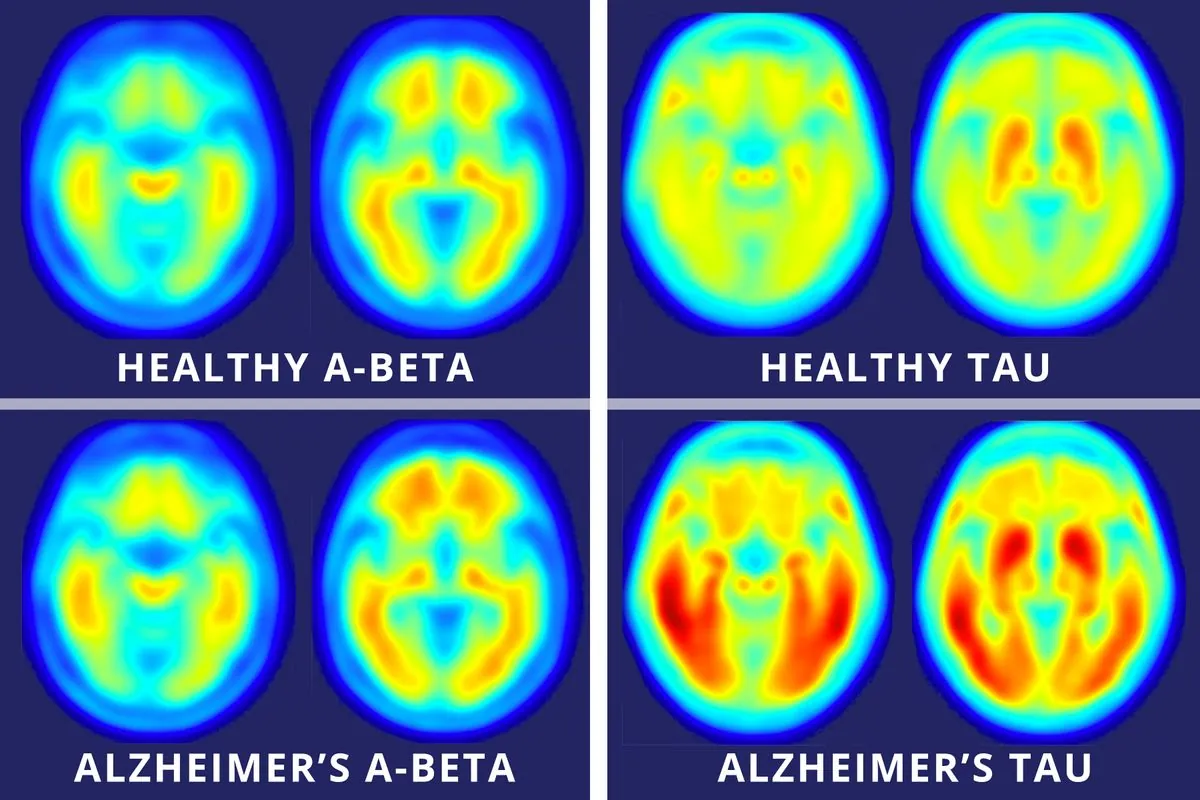
Lecanemab, developed by Japanese pharmaceutical company Eisai, has demonstrated promising results in clinical trials. The drug has been shown to decelerate cognitive decline by 27% in individuals with early-stage Alzheimer's disease. This achievement is particularly noteworthy as it represents the first disease-modifying treatment that not only slows cognitive deterioration but also reduces the characteristic amyloid plaques associated with Alzheimer's.
The Medicines and Healthcare Products Regulatory Agency (MHRA) is expected to grant approval for lecanemab's use in treating early Alzheimer's disease on August 22, 2024. This decision follows the drug's authorization in the United States 19 months ago, in January 2023.

While this development is cause for optimism, it's important to note that lecanemab is not without potential risks. The European Medicines Agency rejected the drug's license application in July 2024, citing concerns about side effects such as brain swelling and bleeding. To mitigate these risks, the MHRA is likely to implement certain exclusions when approving lecanemab.
One key exclusion may involve patients carrying the APOE gene, which is present in approximately 25% of the population. This gene not only increases the risk of developing Alzheimer's but also heightens the likelihood of experiencing amyloid-related imaging abnormalities (Aria), characterized by brain swelling and bleeding. Additionally, individuals taking specific blood-thinning medications may be excluded due to an elevated risk of bleeding.
The effectiveness of lecanemab hinges on early diagnosis, necessitating specialized scans and investigations. This requirement poses a significant challenge for potential NHS rollout, as it would demand a substantial expansion of diagnostic capacity. Currently, only 2.1% of people with dementia undergo the specialized investigations required for such treatments.
"There is no question this is the biggest advance for 30 years. It slows the disease. Slows it by about 25 to 30 per cent. So, you know, maybe if in the old days you were going to get five years before nursing home now you might get seven years before nursing home care."
As of 2024, approximately 982,000 people in the UK are living with dementia, a number projected to reach 1.4 million by 2040. The introduction of lecanemab could potentially alter the trajectory of this devastating disease for many individuals.
It's worth noting that Alzheimer's disease, first described by German psychiatrist Alois Alzheimer in 1906, remains a complex condition. While lecanemab targets amyloid plaques, one of the hallmarks of the disease, Alzheimer's is also characterized by the presence of tau tangles in the brain. The risk of developing Alzheimer's doubles every 5 years after age 65, and women are more likely to be affected than men.
As research continues, it's important to remember that lifestyle factors may play a role in Alzheimer's prevention. Regular physical exercise may reduce the risk by up to 50%, and some studies suggest that certain diets, like the Mediterranean diet, may have protective effects. Additionally, multilingualism has been associated with a 4-5 year delay in the onset of Alzheimer's symptoms.
While lecanemab represents a significant step forward, the journey to fully understand and combat Alzheimer's disease continues. The approval of this drug in the UK marks the beginning of a new chapter in Alzheimer's treatment, offering hope to millions affected by this devastating condition.